Convective temperature gradient#
Let’s visualize what a convectively-unstable temperature gradient looks like. We’ll create two plane-parallel atmospheres: one isothermal (stable against convection) and one isentropic (convectively-unstable).
For plane-parallel, we can take the gravitational acceleration, \(g\), as constant, and write hydrostatic equilibrium as:
Isothermal#
For an isothermal atmosphere, and assuming an ideal gas, we can take:
and inserting this into HSE, we have:
(where we used the fact that \(T\) is constant to pull it out of the derivative. Then rewriting:
where \(H\) is the pressure scale height:
This can be easily integrated to give:
and in terms of pressure,
Isentropic#
If the atmosphere has constant entropy, we can use the adiabatic relation between pressure and density:
Putting this in HSE, we have:
Density#
This can be integrated. We take \(P(r = 0) = P_0\), \(\rho(r=0) = \rho_0\) and find:
we can eliminate \(K\) as \(K = P_0 / \rho_0^{\gamma}\) and get:
or in terms of the scale height, \(H = P_0 / (\rho_0 |g|)\),
Pressure#
Since \(P(r) = K\rho^{\gamma}\), we have:
Temperature#
Finally, if we assume an ideal gas, then
where \(T_0 = \mu m_u P_0 / (k_B \rho_0)\).
The temperature just falls off linearly (for an ideal gas).
Note
If we differentiate this temperature profile, we get:
This is just the adiabatic temperature gradient, \(dT/dr |_\mathrm{ad}\)!
Comparisons#
We can plot these thermodynamic profiles as a function of \(\xi \equiv r/H\)
import matplotlib.pyplot as plt
import numpy as np
xi = np.linspace(0, 5, 100)
gamma = 5./3.
P_isothermal = np.exp(-xi)
P_isentropic = (1 - (gamma - 1)/gamma * xi)**(gamma/(gamma-1))
/tmp/ipykernel_2767/2183992313.py:5: RuntimeWarning: invalid value encountered in power
P_isentropic = (1 - (gamma - 1)/gamma * xi)**(gamma/(gamma-1))
fig, ax = plt.subplots()
ax.plot(xi, P_isothermal, label="isothermal")
ax.plot(xi, P_isentropic, label="isentropic")
ax.set_xlabel("$r/H$")
ax.set_ylabel("$P/P_0$")
ax.legend()
ax.grid(linestyle=":")
fig.savefig("atm.png")
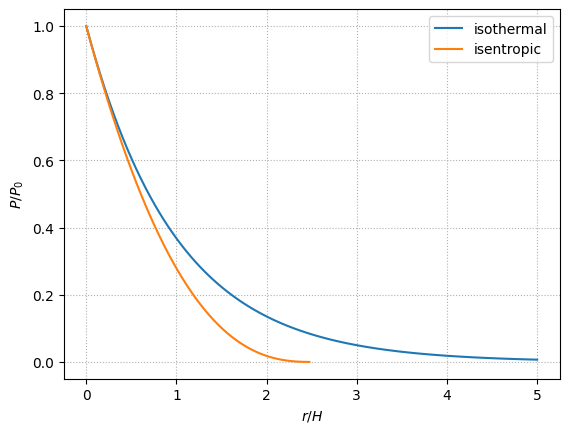
For the same base pressure, the isothermal atmosphere is more extended than the isentropic one.

