Thermodynamics and the Maxwell-Boltzmann distribution#
Here we use SymPy to learn a bit about ideal gases
from sympy import init_session
init_session(use_latex="mathjax")
%matplotlib inline
IPython console for SymPy 1.14.0 (Python 3.11.14-64-bit) (ground types: python)
These commands were executed:
>>> from sympy import *
>>> x, y, z, t = symbols('x y z t')
>>> k, m, n = symbols('k m n', integer=True)
>>> f, g, h = symbols('f g h', cls=Function)
>>> init_printing()
Documentation can be found at https://docs.sympy.org/1.14.0/
Note: we need to tell SymPy that some of these quantities are real and positive so it is able to do the integrals without worrying about complex numbers
ni, m, k, T = symbols("n_I m k_B T", real=True, positive=True)
p, v = symbols("p v", real=True)
n = symbols("n")
Let’s write the Maxwell-Boltzmann distribution, already transformed into spherical coordinates:
our n here includes the \(4\pi p^2\)
n = 4*pi* p**2 * ni / (2 * pi * m * k *T)**Rational(3,2) * exp(-p**2/(2*m*k*T))
n
average velocity#
We want to compute the average velocity:
We’ll express \(v = p / m\) and work in terms of momentum
now switching to spherical coordinates in momentum space:
vavg = (1/ni) * integrate(n * p / m, (p, 0, oo))
vavg
Notice that this value is greater than the most likely velocity (where the peak of the M-B) distribution is. This is because there is a long tail to high velocities. We can see this via a plot
xi = symbols("xi")
We’ll define a dimensionless momentum to allow us to plot this:
Then
with \(n(xi) d\xi\) as
n_dxi = n.subs(p, sqrt(2*m*k*T)*xi)/ni * sqrt(2*m*k*T)
and our average velocity is then:
xi_avg = m * vavg / sqrt(2*m*k*T)
xi_avg
fig = plot(n_dxi, (xi, 0, 5),
markers=[{'args': [[xi_avg], [n_dxi.subs(xi, xi_avg)], "o"]}])
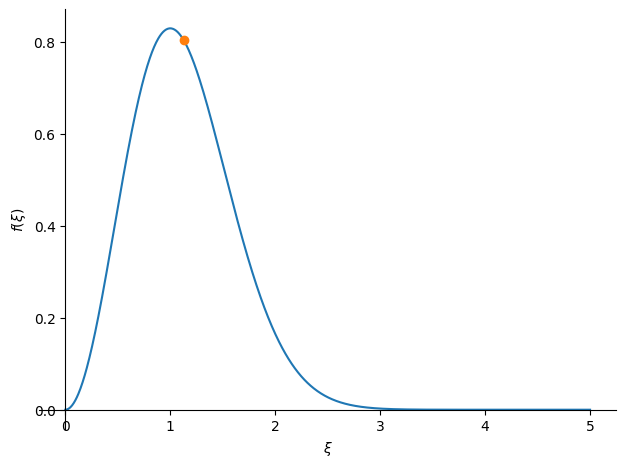
Here we see that the average velocity is slightly larger than the most probably velocity (the location of the peak).

